- The traditional steering committee model of quarterly check-ins and rubber-stamp approvals is being replaced by strategic brain trusts that operate as continuous, agile partners who can quickly convene to turn complex clinical plans into clear action in today's fast-paced environment.
-
Modern steering committees are structured teams of internal and external experts who actively shape strategy throughout the development process, with internal members including medical affairs leaders, clinical development heads, regulatory specialists, and commercial directors, while external members include KOLs, principal investigators, community clinicians, and patient advocates.
-
Early-stage biotechs lack the deep internal teams needed to make high-stakes decisions requiring synthesis of complex clinical information from different sources, and steering committees bridge these gaps by delivering targeted expertise exactly when needed, essentially letting small teams make decisions like large organizations.
-
Steering committees serve seven core functions: scientific strategy and planning to identify meaningful differentiation strategies, protocol development and trial feasibility to pressure-test assumptions, lifecycle and evidence planning to map entire evidence generation strategies, capturing and synthesizing insights using AI-powered analysis, publication and congress planning to guide research prioritization, engaging and building relationships with KOLs through sustained trust rather than transactional interactions, and cross-functional collaboration to ensure all internal stakeholders hear the same expert input simultaneously.
-
Traditional steering committees fail due to operational chaos including communication scattered across Zoom recordings and email threads, documentation nightmares with multiple versions across different systems creating regulatory compliance risks, scheduling complexity that takes weeks to coordinate experts across time zones, compliance risks from using consumer tools like Google Docs for sensitive clinical discussions, and manual inefficiency where medical affairs teams spend hundreds of hours annually on logistics instead of strategy.
-
The "bookend" approach combines an opening synchronous meeting to establish camaraderie and shared context, three weeks of asynchronous discussion where advisors can engage on their own time from different time zones, and a closing synchronous meeting to synthesize learnings, respecting everyone's schedules while building real relationships.
-
Health Expert Connect platform provides a centralized HIPAA-compliant communication hub with one-click access for advisors, real-time multi-user document annotation with automatic version control that maintains complete audit trails, engagement analytics tracking who's participating and how deeply they're diving into materials, built-in compliance features including integrated Sunshine reporting and contract management, and white-glove strategic support including meeting design, professional moderation, and executive summary creation.
-
AI-powered analysis transforms both structured feedback from surveys and polls and unstructured insights from discussions into actionable intelligence, capturing not just what advisors say but how they say it, when they engage, and how their perspectives evolve over time to reveal patterns, sentiment shifts, and emerging themes that might be missed in traditional meeting summaries.
-
A biotech chief medical officer used Health Expert Connect to engage 10 world-leading experts across hepatology, rheumatology, and pulmonary medicine in just six weeks to prioritize indications for three drugs in development, accelerating his development timeline by months compared to traditional approaches, with the bookend model producing cross-pollination insights that wouldn't have emerged from siloed discussions.
-
Unlike larger platforms that require annual commitments and six-figure budgets, ExtendMed offers pilot programs at significantly lower cost, making it perfect for biotechs that need to move fast and prove concepts before scaling, with one client signing a contract within a week and a half of the first conversation because competitors couldn't accommodate a one-off advisory board.
If you’re leading a biotech in 2026, every challenge can feel existential. Breakthrough science is just the start. Turning breakthrough science into actual success means aligning the smartest people in your orbit: internal teams, external experts, clinicians and KOLs.
But when you’re small, nimble, and already working at max bandwidth, working with these busy, brilliant people is like conducting an orchestra mid-flight. Everyone's moving fast, and there's zero room for error.
This is where steering committees come in—when done right.
The "old" steering committee playbook (quarterly check-ins and rubber-stamp approvals) is dying quickly because it's unsustainable and unproductive in today's fast-paced environment. The endless email chains, scattered files and manual logistics are killing momentum exactly when teams need to be building it.
What this traditional model is being replaced with is something that operates more like a strategic brain trust—a team of advisors that can quickly convene to turn a complex clinical plan into clear action.
This guide briefly walks you through our playbook for making steering committees actually work in 2026.
Contact us if you'd like to explore the platform we equip teams with to execute this playbook across their steering committees, advisory boards, and other engagement initiatives within medical affairs. We'll schedule a free, personalized demo to understand exactly what you need and how our platform can enable it.

We built our engagement platform, Health Expert Connect™, to be the single tool where biotechs can orchestrate and run the modern steering committee model.
We provide comprehensive support for emerging biotech companies as they build their medical affairs functions. Given the smaller size of these organizations, it becomes imperative to use external subject matter experts, primary or principal investigators and other leading clinicians effectively.
Our platform offers tools such as document collaboration and discussion boards, enabling steering committees to facilitate publication planning and support for conferences and poster sessions. Investigators can take the lead in authoring manuscripts in development.
Steering committee members can recommend crucial conferences where the organization should present poster sessions. They can also assist in setting up workgroups to develop these poster sessions.
Talk to us to schedule a personalized demo.
What modern steering committees actually do
Before we jump into strategy, let's quickly level-set with the basics.
A productive steering committee today isn't just an advisory panel you "check in" with once in a while. They're structured teams of internal and external experts who actively shape your strategy throughout the development process.
| Who's involved | Internal: Medical affairs leaders, clinical development heads, regulatory specialists, commercial directors External: KOLs, principal investigators, community clinicians, patient advocates |
| What they do | Refine study endpoints Assess recruitment feasibility Align protocols with evolving standards of care Bridge medical, regulatory, commercial and real-world perspectives |
Two real examples
Here are two quick examples to clarify what this looks like in actual practice.
1. Protocol acceleration
A biotech developing a rare autoimmune therapy initially proposes a series of complex patient assessments that would limit enrollment. Its steering committee—KOLs, patient advocates, and community clinicians—recommends practical adjustments: simplified assessment schedules and at-home monitoring. The result: enrollment jumps, trials accelerated by months.
2. Real-world evidence strategy
Another biotech faces payer skepticism about real-world effectiveness. Its committee advises developing parallel RWE collection using digital health trackers alongside traditional trials. This directly addresses payer concerns and improves market access at launch.
“A steering committee is where you say, ‘Let’s actually plan out our next clinical trials.’ It’s not just a forum to discuss ideas—it’s where actionable plans begin. That’s where the right advisors come in. It’s structured, strategic, and it really helps organize all the different moving pieces, whether it’s endpoints, patient recruitment strategies, or post-marketing objectives.”

— Amy Ravi, CEO, ExtendMed
Why emerging biotechs need this now
Early-stage biotechs, by nature of their size, don't typically have the deep internal teams they need to make high-stakes decisions that require synthesizing a lot of complex clinical information from different sources. Every decision has massive downstream implications. A good steering committees bridge these gaps by delivering targeted expertise exactly when needed—essentially letting small teams make decisions like large ones.
For example, let’s say your team is designing a pivotal trial. Without a steering committee, you risk costly, avoidable amendments because crucial practical insights from clinicians weren’t available upfront. A steering committee filled with the right mix of external investigators and clinicians could have helped you foresee enrollment bottlenecks or refine eligibility criteria early, saving time and resources down the line.
Just to break that down again:
| Without a committee: You risk costly trial amendments because you missed practical insights from clinicians upfront. |
With a committee: You foresee enrollment bottlenecks, refine eligibility criteria early, save months and millions. |
These committees also dissolve silos fast. They align clinical, regulatory, commercial and medical affairs into one cohesive strategy. Again, smaller teams get the strategic depth of a much larger organization.
Our platform, Health Expert Connect™ makes this especially powerful for smaller firms who need the cognitive firepower of a much larger firm. One recent client needed flexibility for a six-month pilot with their steering committee—something other engagement platforms couldn't accommodate. That flexibility let them prove the concept before expanding. Read the full story here.
The core functions of a modern steering committee today
 A steering committee does far more than provide periodic input. It acts as a continuous, agile partner across several critical functions. It's important to take a moment to really break this down, since we commonly find that teams struggle to realize all the reasons to convene a powerful steering committee.
A steering committee does far more than provide periodic input. It acts as a continuous, agile partner across several critical functions. It's important to take a moment to really break this down, since we commonly find that teams struggle to realize all the reasons to convene a powerful steering committee.
Let's walk through the functions we help firms take advantage of one by one.
1. Scientific strategy and planning
A steering committee can help you identify meaningful differentiation strategies and areas of unmet need. The right advisors ca help you make sure your development efforts are rooted in clinical relevance and evolving standards of care. For example, we've facilitated committees that've helped pivot from one indication to another based on changes in the competitive landscape or emerging clinical data.
Say you're a biotech developing a novel anti-fibrotic agent. Initial internal discussions might focus on idiopathic pulmonary fibrosis (IPF) as the lead indication. But it's only during steering committee discussions that hepatologists on the panel highlight that the mechanism of action might be particularly relevant for non-alcoholic steatohepatitis (NASH), where existing treatments are limited and regulatory pathways are more favorable.
The committee's cross-disciplinary perspective reveals that targeting NASH first could provide faster regulatory approval, better market positioning, and create a stepping stone to later IPF development—a significant but crucial strategic pivot that internal teams, focused on their original IPF expertise, might never have considered.
Now take it a step further. A steering committee might review your competitive intelligence and identify that while three competitors are pursuing similar mechanisms in your target indication, none are addressing the specific patient subpopulation with comorbid conditions that represents 40% of the market. This insight could lead to a more refined development strategy targeting this underserved segment, creating a defensible market position even in a super crowded field.
2. Protocol development and trial feasibility
Steering committee advisors very often review trial designs, inclusion/exclusion criteria and endpoints—but more importantly, they pressure-test your assumptions. Will that complex patient assessment schedule actually work in community settings? Are your eligibility criteria too restrictive?
Having stood up these committees for many teams, we've found that improving protocols often involves understanding the gap between what is theoretically optimal and what is practically achievable—an insight that requires a specific and thoughtfully arranged panel of experts to determine.
Community physicians often have different patient populations, resource constraints, and workflow limitations compared to academic medical centers, where protocols are typically designed. A really well-constructed steering committee can bridge this gap by providing real-world operational intelligence that prevents costly protocol amendments and enrollment delays.
“Let's imagine your team designed a rheumatoid arthritis trial requiring patients to visit the clinic every two weeks for the first three months, with each visit including specialized imaging, joint assessments and lab work.
The protocol looks scientifically robust on paper. But steering committee members point out that community rheumatologists typically see patients monthly, many patients would need to take time off work for bi-weekly visits, and the specialized imaging requirements would create bottlenecks at community sites that lack dedicated research coordinators.
The committee suggests a modified schedule with telemedicine check-ins between visits and simplified assessments that community sites can realistically execute, potentially improving enrollment by 60% while maintaining scientific rigor. Examples like this are real stories from our clients—and show just how well the right committee can reveal blindspots and 'unknown unknowns.'”

— Amy Ravi, CEO, ExtendMed
Let's take another example: An oncology steering committee might reveal that a planned liquid biopsy collection protocol conflicts with standard chemotherapy administration schedules at most treatment centers, requiring patients to make separate trips.
This insight leads to protocol modifications that align sample collection with routine treatment visits, dramatically improving compliance and reducing patient burden. It's exactly these particular nuances that can have an outsized impact on the results of a trial, but are very hard to predict without a very smart panel of experts at the outset to steer you away from hard-to-predict problems.
You can also approach this from a regulatory perspective: Committee members with regulatory experience can identify endpoints that may seem clinically meaningful but won't satisfy regulatory requirements, helping you avoid late-stage protocol modifications that could delay approval by months or years.
3. Lifecycle and evidence planning
Committees aren't only useful for pivotal trials. Savvy teams also use them to map out their entire evidence generation strategy. They inform post-marketing trial designs, identify real-world data opportunities and help align evidence needs across regulatory authorities, clinicians and payers. This includes advising on pragmatic trial designs, digital health tracker integration and payer-specific evidence requirements.
Different stakeholders require different types of evidence at different times, and steering committees help you anticipate these needs sometimes years in advance. It's exactly this kind of foresight that prevents the kind of reactive, expensive studies firms find themselves trapped in—and creates a cohesive evidence narrative that supports your product throughout its lifecycle.
Committee members might reveal that while your primary endpoint will satisfy FDA requirements, payers in key markets are increasingly focused on healthcare resource utilization and quality-of-life measures. This (non-obvious) insight might push you to incorporate health economics endpoints in your pivotal trials rather than conducting separate, expensive post-marketing studies.
A few other use-cases here worth noting:
- International regulatory alignment: A steering committee with global representation may identify that, while your FDA-focused development plan is solid, European regulators are increasingly emphasizing comparative effectiveness data. Now you can add an active comparator arm to your pivotal trial, satisfying both FDA and EMA requirements with a single study design.
- Digital health integration: Advisors might suggest opportunities to partner with digital health companies to create companion apps that track patient outcomes, medication adherence, and quality of life metrics—generating valuable real-world data while improving patient engagement and clinical outcomes.
4. Capturing and synthesizing insights
The average steering committee generates both structured feedback (surveys, polls) and rich unstructured insights (discussions, debates).
The problem is, without a platform system to capture and synthesize those two kinds of information, teams only capture a fraction of the insights that actually were borne out through the committee.
Our Health Expert Connect™ platform transforms these into actionable intelligence using AI-powered text analysis, visual dashboards and engagement tracking. No more losing valuable insights in the translation from discussion to action. Modern platforms capture not just what advisors say, but how they say it, when they engage, and how their perspectives evolve over time. This creates a rich intelligence layer that reveals patterns, sentiment shifts, and emerging themes that might be missed in traditional meeting summaries.
Here's an example of this in practice:
- During a steering committee discussion about dosing strategies, an AI-powered analysis identifies that cardiologists consistently use more cautious language ("might consider," "potentially") when discussing higher doses, while endocrinologists express greater confidence ("should definitely," "clearly beneficial").
- This subtle sentiment analysis reveals specialty-specific comfort levels that weren't apparent in the surface-level discussion.
- The platform's engagement tracking shows that cardiologists spend significantly more time reviewing safety data sections, while endocrinologists focus on efficacy outcomes.
These insights inform specialty-specific educational materials and help anticipate adoption patterns by medical specialty.
Again, we also use our platform for tracking how advisor sentiment evolves as they're exposed to new data over time. You might notice that initial skepticism about your mechanism of action gradually shifts to enthusiasm as advisors review emerging competitive data, providing early indicators of market reception.
On top of all of this, we're able to analyze language patterns and engagement metrics across multiple advisory boards to identify early warning signs of potential adoption challenges or emerging enthusiasm for unexpected applications of your therapy.
With the smart and appropriate integration of AI, we're finally capturing every insight a panel generates in ways teams often don't even know is possible.
5. Publication and congress/conference planning
A committee can guide more than just abstract submissions. We've convened them to help prioritize research topics, identify the right authors, shape data visualization strategies and refine messaging for different audiences.
For example, you can lean on advisors to help craft a publication narrative that builds scientific credibility, addresses key stakeholder concerns and positions your therapy within the broader treatment landscape. We find that the right members can understand the subtle but critical differences between what resonates with academics versus practicing physicians versus regulatory reviewers.
Here are a few specific use-cases to consider:
- Author strategy development: Committee members help identify emerging thought leaders who could become powerful advocates for your therapy. They might suggest including younger investigators as co-authors to build relationships with the next generation of clinical leaders.
- Improving data visualizations: Advisors provide feedback on how to present complex data in ways that resonate with different audiences. What impresses academic researchers might overwhelm community physicians, and what satisfies regulatory reviewers might bore practicing clinicians.
- Congress event engagement: Using QR codes on poster presentations to link to ongoing steering committee discussions, allowing congress attendees to provide real-time feedback and continue conversations beyond the meeting. This creates a feedback loop that informs future research directions and builds broader clinical engagement.
- Thought leadership development: The committee helps identify research gaps and controversial topics where your company could establish thought leadership through investigator-initiated studies or symposium presentations.
6. Engaging and building relationships with KOLs
Rather than one-off advisory boards, steering committees build sustained trust with influential experts. It's a great format for future partnerships, speaker programs and advocacy.
“Traditional advisory boards often feel very transactional—experts provide input, get paid, and move on. Steering committees create ongoing partnerships where experts become genuine stakeholders in your success.
It's a more 'sustained' engagement model that turns advisors from consultants into actual advocates who have intellectual ownership in your development strategy.

— Amy Ravi, CEO, ExtendMed
Here's an example to illustrate:
Rather than establishing separate advisory boards for different indications, you establish a cross-functional steering committee that meets quarterly over a two-year period. Members include hepatologists, rheumatologists, and pulmonologists working on your anti-fibrotic therapy.
Over time, committee members begin collaborating on investigator-initiated research projects, co-authoring publications, and referring patients to each other's studies. The hepatologist on your committee becomes intrigued by the rheumatology data and initiates a pilot study of your therapy in liver fibrosis.
Meanwhile, the pulmonologist collaborates with the rheumatologist on a paper about fibrosis biomarkers across organ systems. These organic collaborations generate additional clinical data, expand your evidence base, and create a network of clinical champions who have genuine scientific investment in your program.
Here are three specific dimensions of this we've built steering committees for:
- Developing advocacy: Long-term steering committee members can become natural advocates because they've shaped your development strategy from the beginning. When they present at conferences or discuss your therapy with colleagues, they're not just endorsing your product—they're defending scientific decisions they helped make.
- Pipeline development: Sustained relationships with steering committee members can open up opportunities for future collaboration on next-generation therapies. Have them provide early feedback on new mechanisms, help identify promising research directions and offer continuity across your development portfolio.
- Peer influence networks: Steering committee members often influence their colleagues through informal networks that extend far beyond formal presentations. A respected committee member's casual comment about your therapy at a medical meeting might carry more weight than a sponsored symposium.
7. General cross-functional collaboration
One of the biggest challenges in drug development is ensuring that different internal teams are working from the same expert insights. Medical affairs might hear one set of feedback, clinical development another, and commercial teams yet another perspective. This leads to inconsistent strategies and missed opportunities for synergy. Unified steering committee platforms ensure all internal stakeholders hear the same expert input simultaneously, creating natural alignment around shared insights.
“During a steering committee discussion about your novel diabetes therapy, experts might express enthusiasm about the efficacy data but raise concerns about dosing complexity in elderly patients. Your medical affairs team hears this as a need for physician education programs. Your clinical team recognizes an opportunity to design a geriatric-focused sub-study. Your regulatory team identifies a potential label optimization strategy. Your commercial team sees a market segmentation opportunity.
Instead of these insights being filtered through different lenses and potentially conflicting interpretations, the unified platform ensures everyone starts with the same expert feedback. The result is a coordinated response: clinical designs the geriatric sub-study, medical affairs develops targeted educational materials, regulatory prepares a label strategy that addresses dosing concerns, and commercial creates market positioning that acknowledges the complexity while emphasizing the therapy's benefits. We see this kind of coordination prevent mixed messages and creates a more powerful, unified approach to market development.

— Amy Ravi, CEO, ExtendMed
We tend to see a few advantages emerge here that aren't always obvious or expected, but super important nonetheless:
- Real-time clarifications: When internal teams have questions about expert feedback, they can ask for clarification directly during ongoing committee discussions rather than relying on secondhand interpretations that might introduce bias or misunderstanding.
- Better strategic alignment: Different departments often have competing priorities and interpretations of market feedback. When everyone hears the same expert insights simultaneously, it becomes harder to justify strategies that contradict clear advisor guidance.
- Efficiency gains: Instead of medical affairs conducting separate KOL interviews, clinical teams running independent feasibility assessments, and commercial teams commissioning separate market research, steering committees provide comprehensive insights that serve multiple internal stakeholders simultaneously.
- Faster, better decision-making: When cross-functional teams are aligned on expert input, decision-making becomes faster and more confident. There's less time spent debating the validity of different data sources or reconciling conflicting external insights.
“I always urge teams to think of the steering committee as a strategic engine, not a checkpoint. With the right advisors and tools, it becomes continuous feedback, structured iteration and long-term alignment.

— Amy Ravi, CEO, ExtendMed
The problems that typically kill steering committees
Despite their value, most traditional steering committees fail (at least to some degree) due to the operational chaos that diverts focus from strategic work when everything is managed in different systems that don't talk to one another.
Here's what we typically see and hear about when pharma teams come to us looking for a system solution:
| Communication chaos | Critical information gets scattered across Zoom recordings, email threads with 50+ replies and shared drives with inconsistent naming conventions. When one advisor's crucial protocol suggestion gets buried in email thread #47, you risk a costly amendment six months later. |
| Documentation nightmares | Without a single source of truth, you're managing multiple document versions across different systems. Version control becomes impossible—is the protocol feedback in v3.2 or v3.2_FINAL_FINAL? This isn't just frustrating; it creates regulatory compliance risks when you can't produce a clear audit trail. |
| Scheduling complexity | Coordinating 10 experts across 5 time zones for quarterly meetings? You'll spend weeks on scheduling polls, calendar invites and rescheduling when conflicts arise. Meanwhile, critical decisions wait. One CMO came to us after spending three weeks just trying to schedule an opening meeting that everyone could attend. |
| Compliance roulette | Using consumer tools like Google Docs or Dropbox for sensitive clinical discussions? You're one audit away from serious problems. Add in Sunshine reporting requirements, contract tracking and payment documentation—manual processes multiply your risk. |
| Manual inefficiency | Your medical affairs team spends days creating meeting agendas, sending reminders, taking notes, consolidating feedback from separate document copies and preparing executive summaries. That's hundreds of hours annually on logistics instead of strategy. As one client told us, their chief medical officer was drowning in administrative tasks when he should have been focusing on the science. |
| Resource drain | When your lean team manages steering committees manually, something has to give. Either the committee gets less attention (reducing its value) or other strategic initiatives suffer. |
One biotech we worked with spent three weeks consolidating protocol feedback because eight advisors each annotated different document versions, some via email, others via tracked changes. After implementing Health Expert Connect™, the same process took two days with automatic version control and centralized feedback.
The technology solution
Digital tools like Health Expert Connect™ now solve these persistent challenges, but not all platforms are created equal.
Here's a quick breakdown of the components of our platform that make teams most excited to use it for ad boards and steering committees.
1. Centralized communication hub
Health Expert Connect™ integrates all communication channels into one HIPAA-compliant platform. Your advisors get one-click access via email or text—they click and they're in, whether joining a Zoom meeting, contributing to a discussion board or completing a survey.
Everything is captured centrally, eliminating the chaos of scattered information. As one project manager told us recently: "Advisors love that they can dictate their responses directly into the discussion board, just like they're used to dictating clinical notes."
2. Hybrid engagement models
We often help clients run our "bookend" approach to ad boards and steering committees: opening and closing synchronous meetings with three weeks of asynchronous discussion in between.
This model respects everyone's schedules while building real relationships. Advisors meet face-to-face virtually to establish camaraderie, then dive deep into discussions on their own time—whether that's midnight in Tokyo or early morning in London.
 One CMO using this approach engaged 10 world-leading specialists across rheumatology, hepatology and pulmonology in just six weeks, accelerating his development timeline by months.
One CMO using this approach engaged 10 world-leading specialists across rheumatology, hepatology and pulmonology in just six weeks, accelerating his development timeline by months.
3. Smarter document collaboration
Real-time, multi-user annotation with automatic version control eliminates the nightmare of "protocol_v3_FINAL_John_edits_REVISED.docx" confusion.
Every comment is tagged to the reviewer, time-stamped and preserved in Health Expert Connect™. Advisors see each other's feedback in real-time, building on ideas rather than duplicating efforts. The platform maintains a complete audit trail of who changed what and when, critical for regulatory compliance.

4. Engagement analytics and tracking
Know exactly who's participating and how. Our platform tracks document views, discussion contributions, meeting attendance and survey completion automatically. You can see which advisors are diving deep into materials versus just showing up.
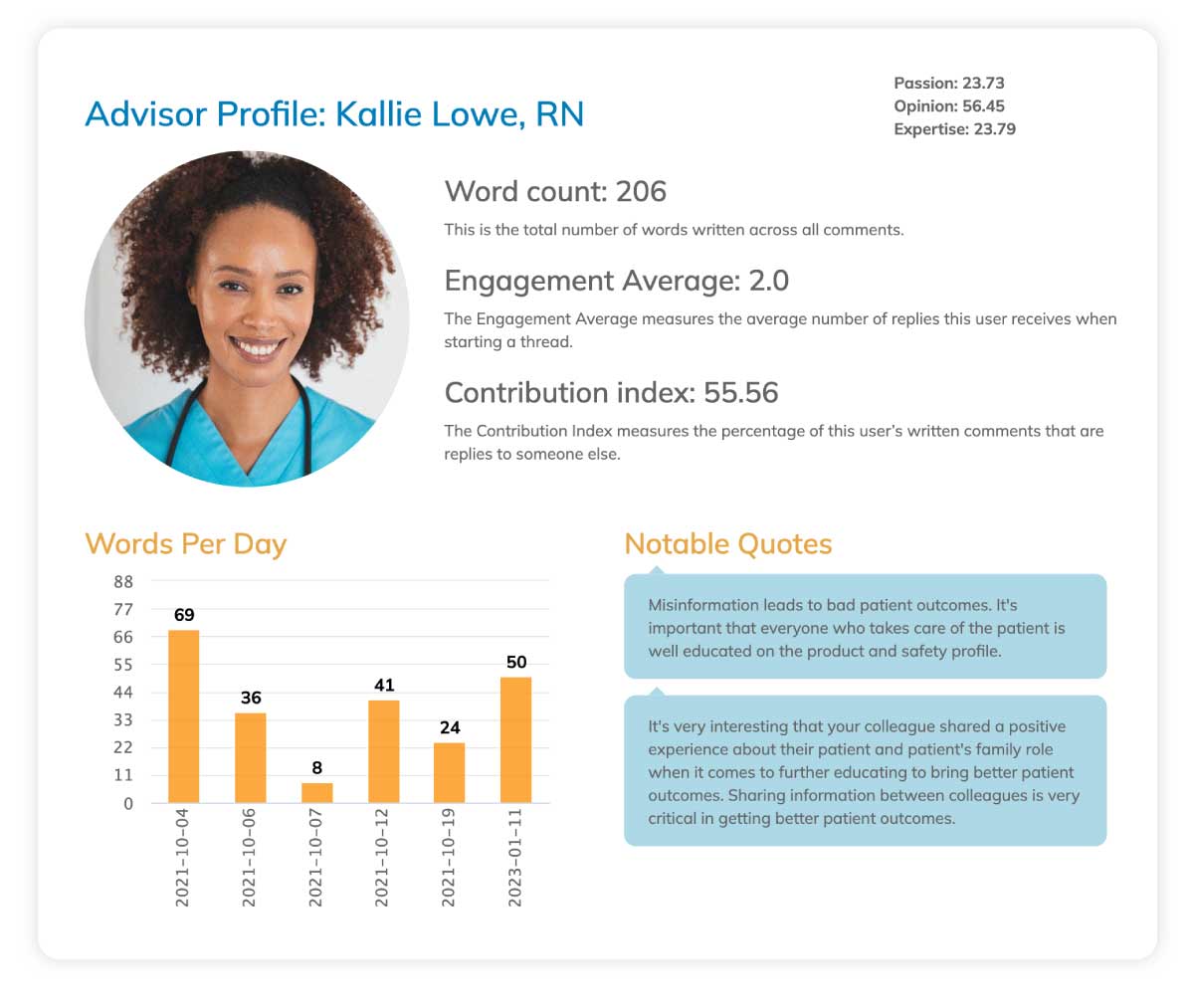 This insight helps you identify your most engaged experts and nurture those relationships. Plus, it creates documentation showing meaningful advisor involvement, not just payment records.
This insight helps you identify your most engaged experts and nurture those relationships. Plus, it creates documentation showing meaningful advisor involvement, not just payment records.
5. Built-in compliance
Health Expert Connect™ was designed for life sciences compliance from day one—not retrofitted consumer software.
Features include secure data storage with encryption at rest and in transit, integrated Sunshine reporting that automatically tracks payments and transfers, complete audit trails showing every interaction, and contract management with expiration alerts. Everything you need for regulatory confidence in one system.
6. Strategic support and best practices
Technology alone isn't enough—especially for small biotechs who need strategy, too. Our team provides hands-on support that surprises even experienced steering committee managers. We help design meeting agendas, create slide decks, moderate discussions and—critically—produce executive summaries after each engagement.
One chief medical officer told us: "I had something ready to take to my board immediately after each meeting. That level of service transformed how quickly we could move."
A Quick Case Study
 The Challenge
The Challenge
The chief medical officer at a biotech developing fibrosis treatments needed to prioritize indications for three drugs in development. Having used Within3 at his previous big pharma role, he initially wanted to work with them again. But they couldn't accommodate his need for a one-off advisory board. He found ExtendMed through our comparison page and signed a contract within a week and a half of our first conversation.
Our Approach
This CMO came to us wanting just an asynchronous discussion. We convinced him to try our bookend model: opening meeting to build relationships, three weeks of asynchronous discussion for a deep dive and closing meeting to synthesize learnings. We guided him to keep all three specialties together as a cross-functional board rather than siloing them. "Advisors really love that," we explained, and one participant later confirmed: "The highlight was having experts from different areas—discussions were enriched by contributions from different disease areas."
Unexpected Value
Beyond the platform, the CMO was surprised by our service level. Our team created his slide deck, proposed the agenda and timing, prepared warm-up slides and managed all logistics. After each phase, we delivered executive summaries he could immediately share with his board and management team. He loved working with our team and felt like he had a real strategic partner.
Results
A starter package fit his budget perfectly (versus six-figure annual contracts elsewhere).
6 weeks total from kickoff to final insights.
10 world-leading experts engaged across hepatology, rheumatology and pulmonology.
Cross-pollination insights that wouldn't have emerged from siloed discussions.
Executive-ready summaries after each engagement phase.
Expansion opportunities—the team is now exploring how to use Health Expert Connect™ for congress feedback via QR codes on poster presentations.
In a matter of six weeks, he met with world leaders in three different therapeutic areas—all principal investigators on numerous studies. As the lone chief medical officer, he catapulted his business forward with insights that would be near-impossible to gather alone.
Your next steps
Whether launching your first committee or scaling for a growing pipeline, ExtendMed provides both the technology and expertise to make steering committees work.
Health Expert Connect™ delivers comprehensive functionality:
- Flexible engagement models: Synchronous meetings, asynchronous discussions, surveys—use what works for your advisors
- Meeting orchestration: Automated scheduling (we survey advisors to find optimal times), agenda templates, reminder sequences and seamless Zoom integration
- Smart content management: Custom-branded libraries, version-controlled documents, real-time collaborative annotation and role-based access
- Powerful analytics: AI-powered transcription, sentiment analysis, engagement tracking and automated executive summaries
- Complete compliance: HIPAA-compliant infrastructure, integrated Sunshine reporting, full audit trails and contract management
- White-glove service: Strategic guidance, meeting design, professional moderation and executive summary creation
Unlike larger platforms that require annual commitments and six-figure budgets, we offer pilot programs starting far lower. Perfect for biotechs that need to move fast and prove concepts before scaling.
Stop letting logistics drain your team's energy. Transform your steering committees from administrative burdens into strategic accelerators that drive faster trials, sharper evidence strategies and stronger launches.
Our goal is to help you have richer and more frequent engagements with each of your stakeholders, at a lower cost. How long are you going to wait to solicit better insights across your programs?
Visit our solutions page for a quick look at our solutions and how we empower teams to have richer engagements with each of their stakeholders. Want to get in touch with questions or schedule a free platform demo? Contact us or submit the form below to share a little about yourself and your goals. We'll be in touch within one business day.





