Steering Committee Management
Organize communication and collaboration among external SMEs, principal investigators and leading clinicians
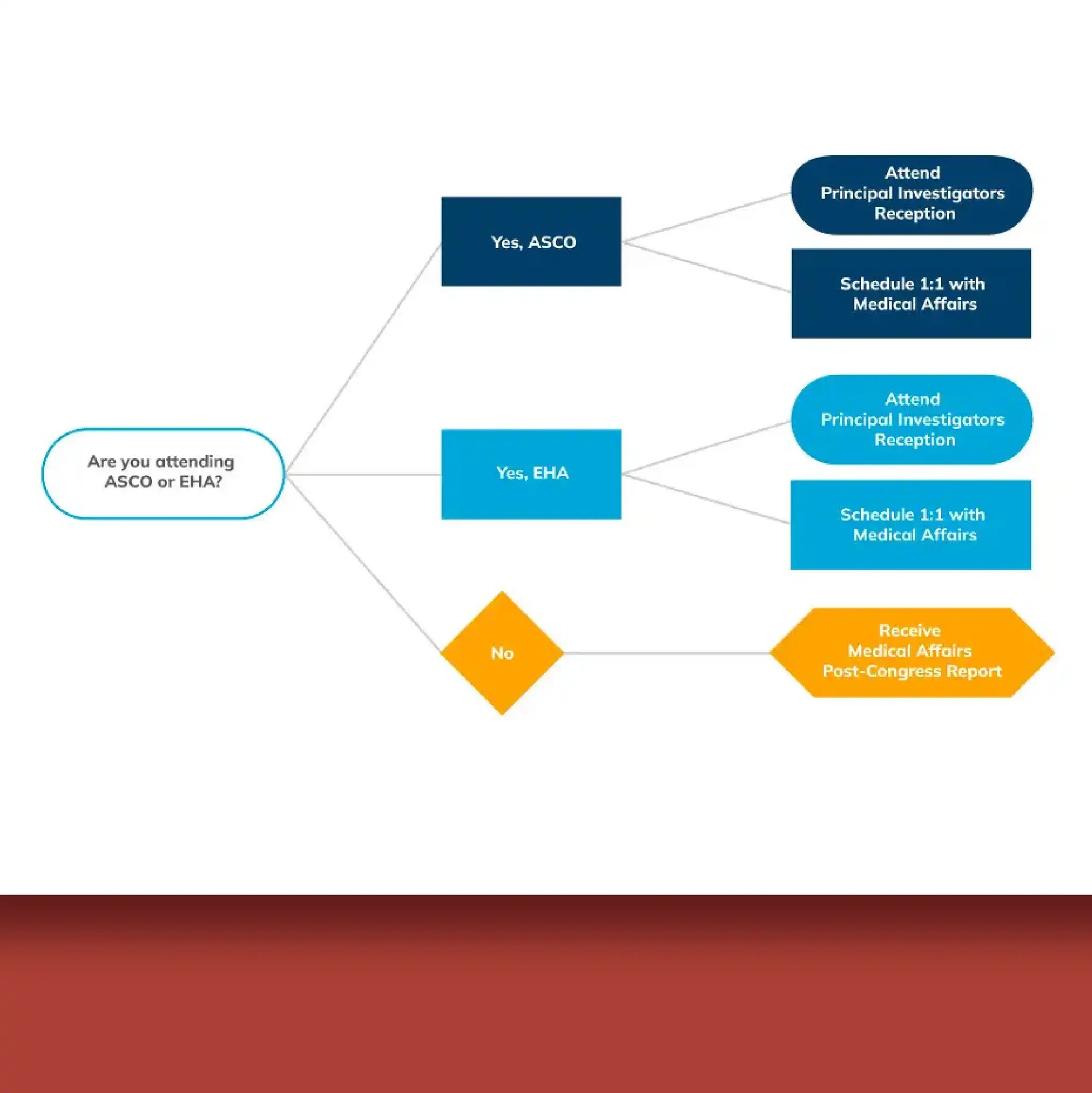
Whether you need comprehensive steering committee support or targeted collaboration tools, ExtendMed's Health Expert Connect™ provides a centralized system to streamline operations, enhance collaboration, and drive productive outcomes for smaller biotech medical affairs teams.